Explainer: Johnstone's Triangle
Alex Johnstone was a leading figure in post-war British Chemistry Education. He worked as a Chemistry teacher in Scotland from the late 1950s before moving into HE roles within the University of Glasgow from the late 1960s. He worked on a lot of areas, from secondary curriculum design to University exam outcomes.
One of his contributions was a ‘triangle’ describing the difficulty of learning chemistry. Its value is probably in how effectively it compresses sophisticated educational research into a versatile model which teachers can use practically.
What is the triangle?
The triangle can be seen as describing ‘mastery’ of a chemical phenomenon. To really understand what’s going on, a chemist must be able to think about things using three perspectives: macroscale, sub-microscale, and symbolic.
A triangular representation of Johnstone’s three levels of chemical reasoning.
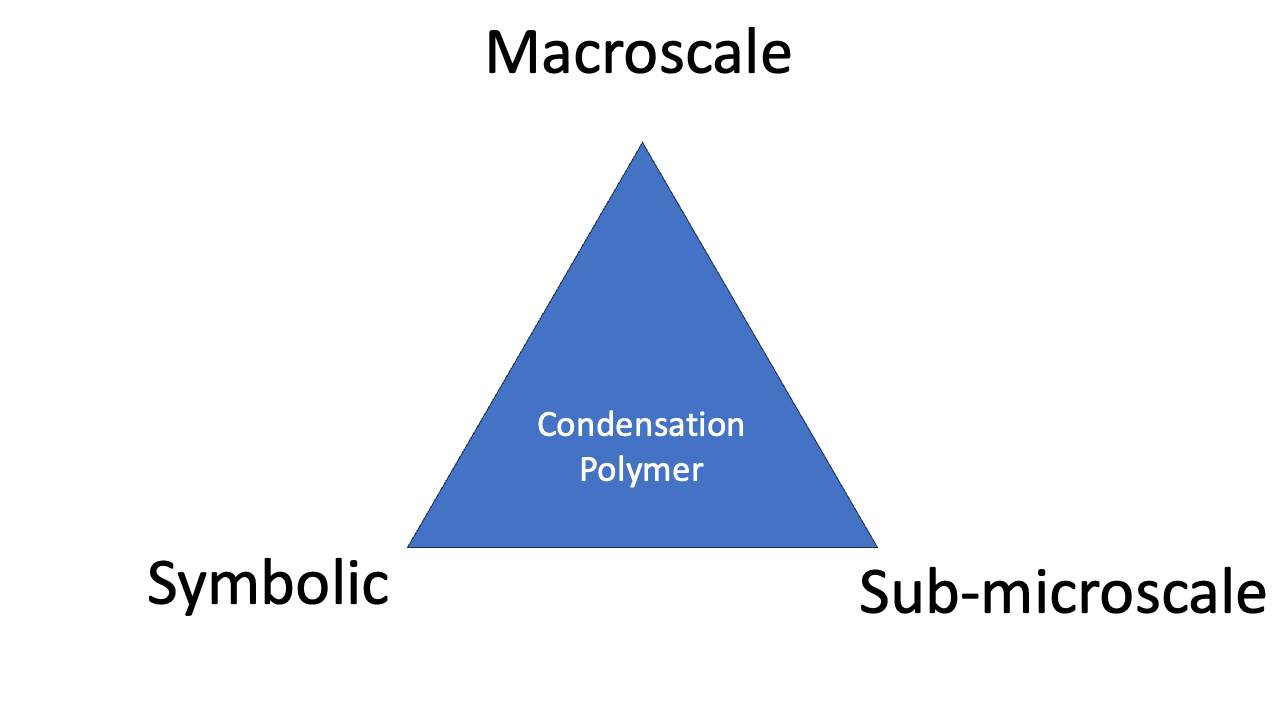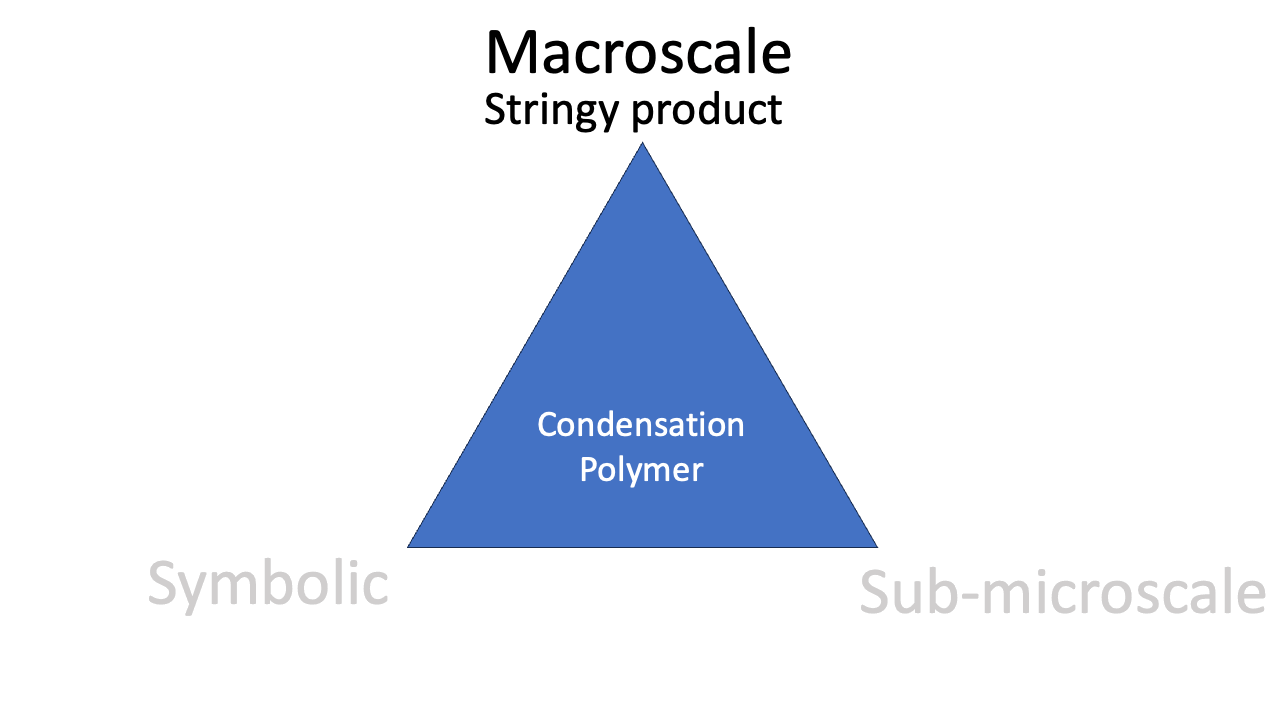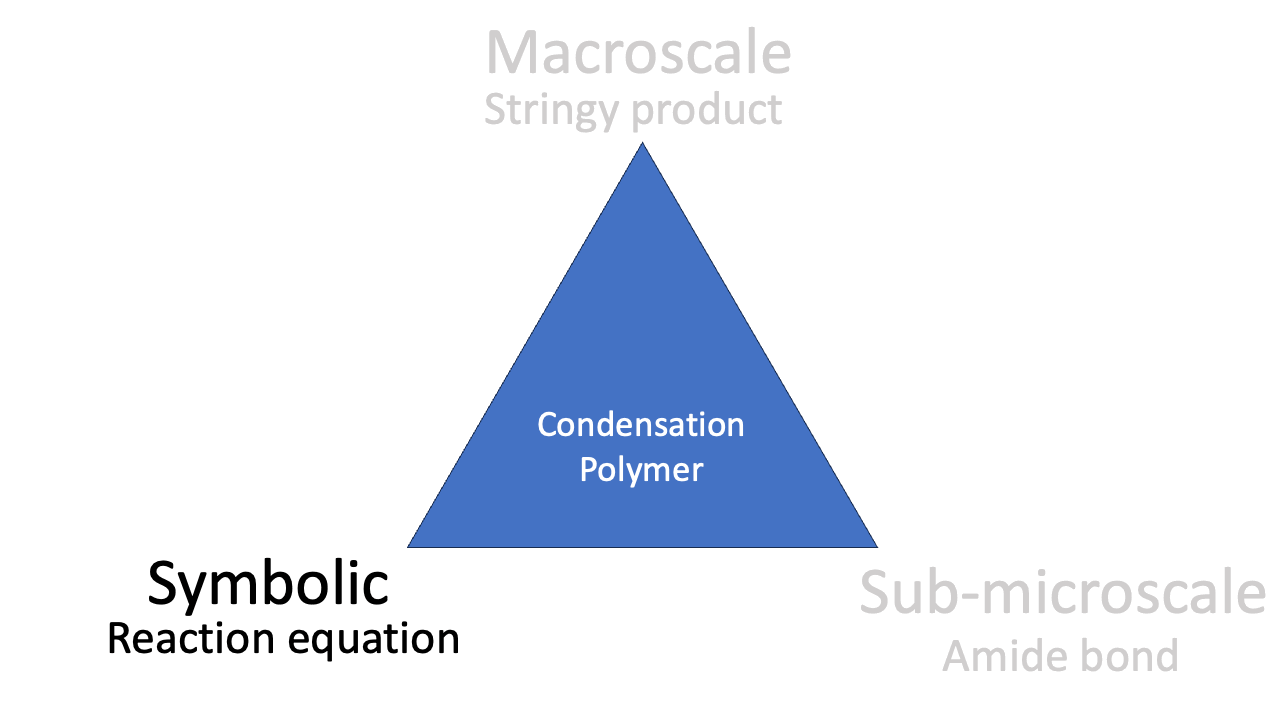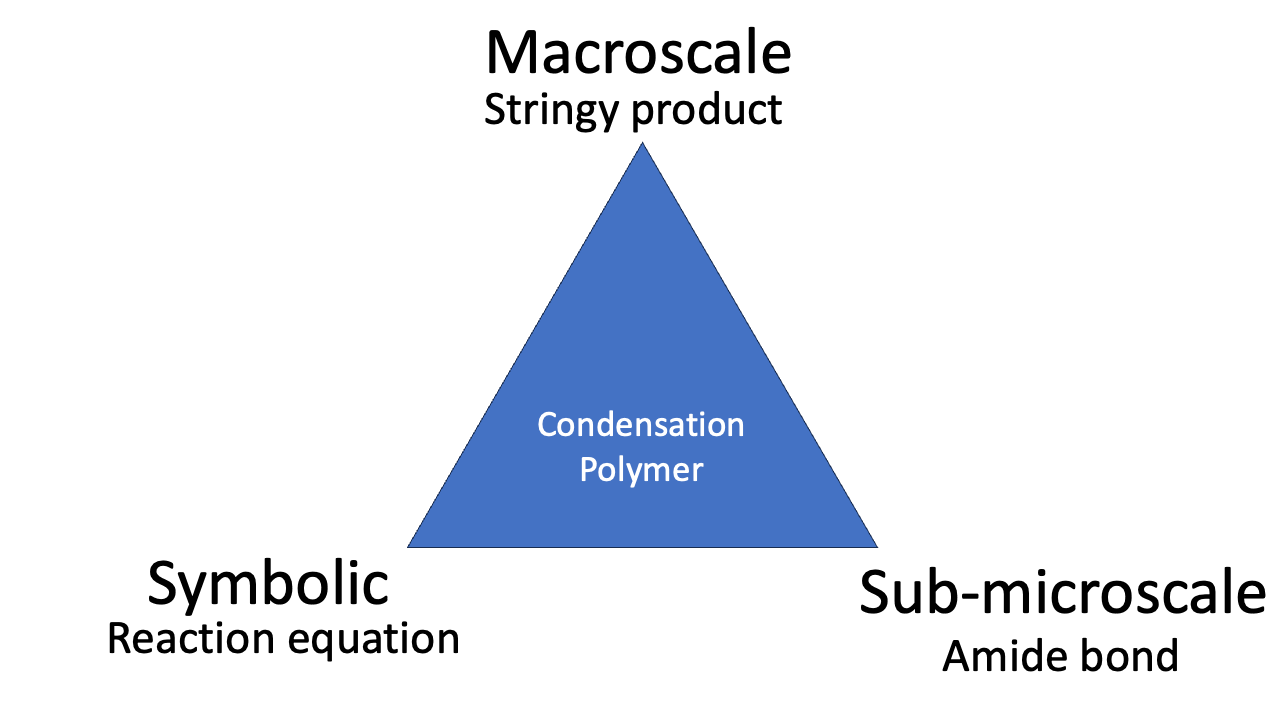
Examples help to explain what the triangle is talking about. So, how does Johnstone’s Triangle treat (i) the ligand field splitting of a transition metal complex; and (ii) the synthesis of the condensation polymer Nylon?
Macroscale
The observable phenomenon of the colour of a complex is a good example of a macroscale perspective. “Aqueous copper(II) is blue” is a macroscale observation. The stringiness of a polymer is an observable aspect of the polymer. The classic “reel” lab demonstration is a popular way to convey this macroscale idea.
Sub-microscale
The geometry of the aqueous copper(II) complex might be a piece of sub-microscale knowledge. The structure of the amide bond in Nylon is also a sub-microscale idea. In both the copper and the polymer cases, these sub-microscale considerations are structural.
Symbolic
The splitting diagram of the d-orbitals in copper(II) is a symbolic representation, as is the reaction equation for the condensation polymerisation. Symbolic representations often appeal quite closely to the theories and models used by chemists. This can mean that mathematical notation and graphs become important symbolic representations in some contexts (see kinetics paper below).
Putting it all together
The phenomenon of blue Cu(II) is related to the field splitting associated with the complex’s structure. A satisfying grasp of this piece of chemistry embraces all three corners of Johnstone’s Triangle.
The phenomenon of condensation polymerisation is related to the reaction equation (“look: we *condense* water”), the representation of the molecular structure, and the observable stringiness of the reaction product. Mastering the idea of condensation polymerisation involves all three corners of the triangle.
What’s the point of the triangle?
I’ve seen the triangle used in two senses: to describe mastery and to scaffold effective teaching. The mastery argument is that a chemist who can freely switch between the corners of the triangle has understood the phenomenon. The scaffolding argument is that coming at the phenomenon from several perspectives can help students to ‘get’ what’s going on.
While I think the mastery argument explains that sense of satisfaction which often characterises meaningful learning, I find the scaffolding argument more persuasive. At heart, the triangle is a response to the enormous cognitive load which Chemistry exerts upon learners. Arguably, it pushes educators into patterns of teaching which minimise extraneous load. This emphasis is really helpful, particularly for people looking to begin or renew their teaching.
Criticisms
I feel very ungrateful voicing criticisms, because I think on balance the triangle has served Chemistry educators really well. At the same time, I think it’s worth thinking about where it stops being an appropriate model for teaching.
HE and the Macroscale
In my view Higher Education basically ditches the macroscale, at least in the theoretical coverage embodied in lecture courses. This means it often provides very little help if you want to teach advanced concepts. This is often compounded by the quantum mechanical basis of much of the science (our everyday experience is much more classical than quantum) and the classical I/O/P distinction squeezing out Analytical Chemistry as a systematic discipline studying the acts of observation and measurement.
The teaching lab is an interesting thing to think about in the context of the macroscale representation. Practical chemistry is often very concerned with the observable world (the rotavap getting cold, a TLC spot matching your product), and it might be one space where the triangle has scope to be applied really meaningfully.
HE Mastery is (sometimes) Pluralistic
I wonder if this is perhaps strongest in Inorganic Chemistry, but I’ve become increasingly convinced that true mastery involves a sincerely pluralistic view of the symbolic representation of phenomena. To use both VSEPR and MO theory to describe the linear/bent comparison between CO2 and O3 is a really good thing. To pursue an ionic account of bonding in AgCl before complicating it with covalency is an enriching experience. There is a (hermeneutic) value to exploring many ways of seeing the same thing.
Even when there is a single dominant theoretical representation, it is joyful to be challenged with the different perspectives of students: a plausible-but-wrong argument or an unusual emphasis can make for a rich discussion which teases out things that are educationally valuable. I think that mastery is about that, too: reasoning your way through your own misconceptions is an incredibly important skill for a scientist.
Conclusion
Perhaps Johnstone’s Triangle could be adapted somehow to accommodate a smaller amount of macroscale reasoning (see the Taber reference below) or pluralistic reasoning, but maybe it’s just as valuable to see that this shouldn’t be seen as a completely comprehensive model. There is great value in it, but that value is probably greatest for the situation it was designed around: secondary-school content.
I have found it useful as a prompt for thinking about how to make lectures more interesting (could I… make a complex for students in each lecture?) and to try and push some of my tutorial questions into macroscale considerations (why does a metal feel cold when you touch it?). This has improved my teaching! Thanks, Johnstone!
But the bigger picture is that Johnstone’s Triangle is an attempt to simplify his insight that the great risk in teaching Chemistry is cognitive overload. The triangle is one way to try and manage the radically-limited capacity of students to process new information. There are lots of other ways to do this, but to have one way mapped out so clearly is a really useful thing (particularly for people new to teaching).
Note
There is currently no Wikipedia article on Johnstone’s Triangle. It feels like we could do something about that.
Further Reading
A superb scholarly reflection on the triangle by Kieth Taber. A useful tour of the history of the construction, and some discussion of what the edges of the triangle might be seen as doing.
An EiC article discussing how the triangle might be used when modelling concepts to pupils.
Using the triangle to design flipped instruction in HE.
The topic of kinetics mapped onto the triangle (really good for exploring mathematical and graphical representations as symbolic ideas).